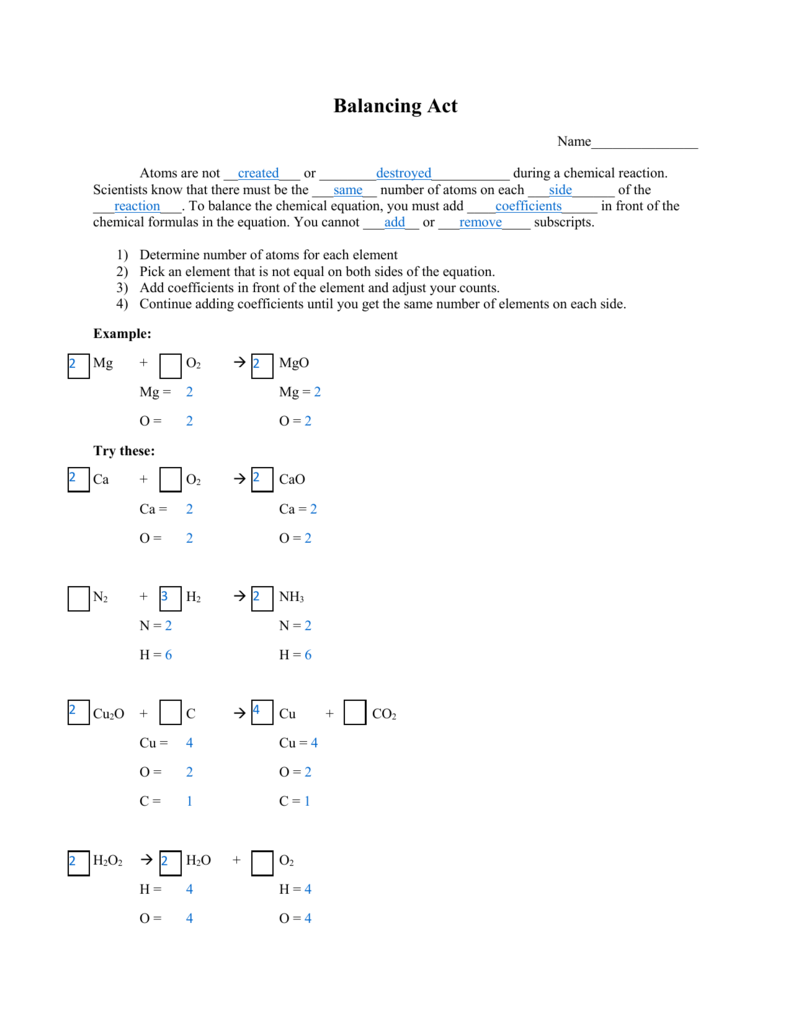
Balancing equations is a fundamental skill in chemistry that involves ensuring that the same number of each type of atom is present on both sides of a chemical equation. This process can be challenging for students to grasp, but with practice, it becomes easier to understand and master. One of the best ways to practice balancing equations is through the use of worksheets that provide a series of equations for students to balance.
These worksheets typically include a mix of simple and more complex chemical equations, allowing students to gradually build their skills and confidence in balancing equations. The answer key for these worksheets is an essential tool that allows students to check their work and identify any mistakes they may have made. By using the answer key, students can learn from their errors and improve their understanding of the balancing process.
Balancing Equations Practice Worksheet Answer Key
One common type of balancing equations practice worksheet includes equations such as:
- H2 + O2 → H2O
- Fe + O2 → Fe2O3
- Al + Cl2 → AlCl3
Using the answer key provided, students can verify that they have correctly balanced each equation by ensuring that the number of atoms of each element is the same on both sides of the equation. This allows students to practice and reinforce their understanding of the principles of conservation of mass and the Law of Definite Proportions.
As students work through the practice worksheet and check their answers against the answer key, they can also begin to identify patterns and strategies for balancing equations more efficiently. By recognizing common elements and compounds and understanding how to manipulate coefficients and subscripts, students can develop a systematic approach to balancing equations that will serve them well in more complex chemical reactions.
In conclusion, practicing balancing equations using worksheets and answer keys is a valuable tool for students to improve their understanding of this essential chemistry skill. By working through a variety of equations and checking their answers against the answer key, students can build their confidence and proficiency in balancing equations. This practice will not only benefit students in their chemistry studies but also in future pursuits that require a strong foundation in chemical principles.