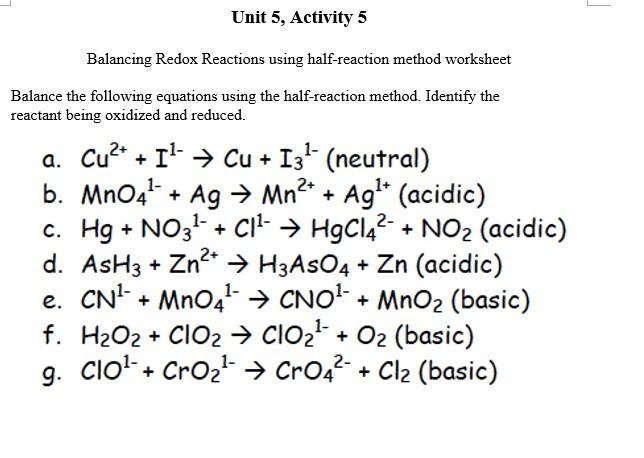
Redox equations are chemical equations that involve the transfer of electrons between atoms or ions. These types of reactions are essential in various chemical processes, including oxidation, reduction, and combustion. Understanding redox equations is crucial in chemistry as it helps in predicting the behavior of substances and their interactions with each other.
A redox equations worksheet is a helpful tool for students to practice balancing and solving redox equations. This worksheet typically contains a series of redox reactions that students need to balance by adjusting the coefficients of the reactants and products. By working through these equations, students can improve their understanding of redox reactions and enhance their problem-solving skills.
When working on a redox equations worksheet, it is important to remember the following key concepts:
- Identify the oxidation and reduction half-reactions in the equation.
- Balance the atoms in each half-reaction by adding the appropriate coefficients.
- Balance the charge by adding electrons to one or both half-reactions.
- Ensure that the number of electrons lost in the oxidation half-reaction equals the number of electrons gained in the reduction half-reaction.
- Combine the balanced half-reactions to obtain the overall balanced redox equation.
Practice makes perfect when it comes to balancing redox equations, so working through multiple examples on a redox equations worksheet can help students master this skill. By practicing regularly, students can become more confident in identifying redox reactions, balancing equations, and understanding the underlying principles of electron transfer in chemical reactions.
In conclusion, a redox equations worksheet is a valuable resource for students studying chemistry. By providing a series of redox reactions to balance and solve, this worksheet allows students to practice and improve their skills in balancing redox equations. Through consistent practice and application of key concepts, students can enhance their understanding of redox reactions and develop their problem-solving abilities in chemistry.