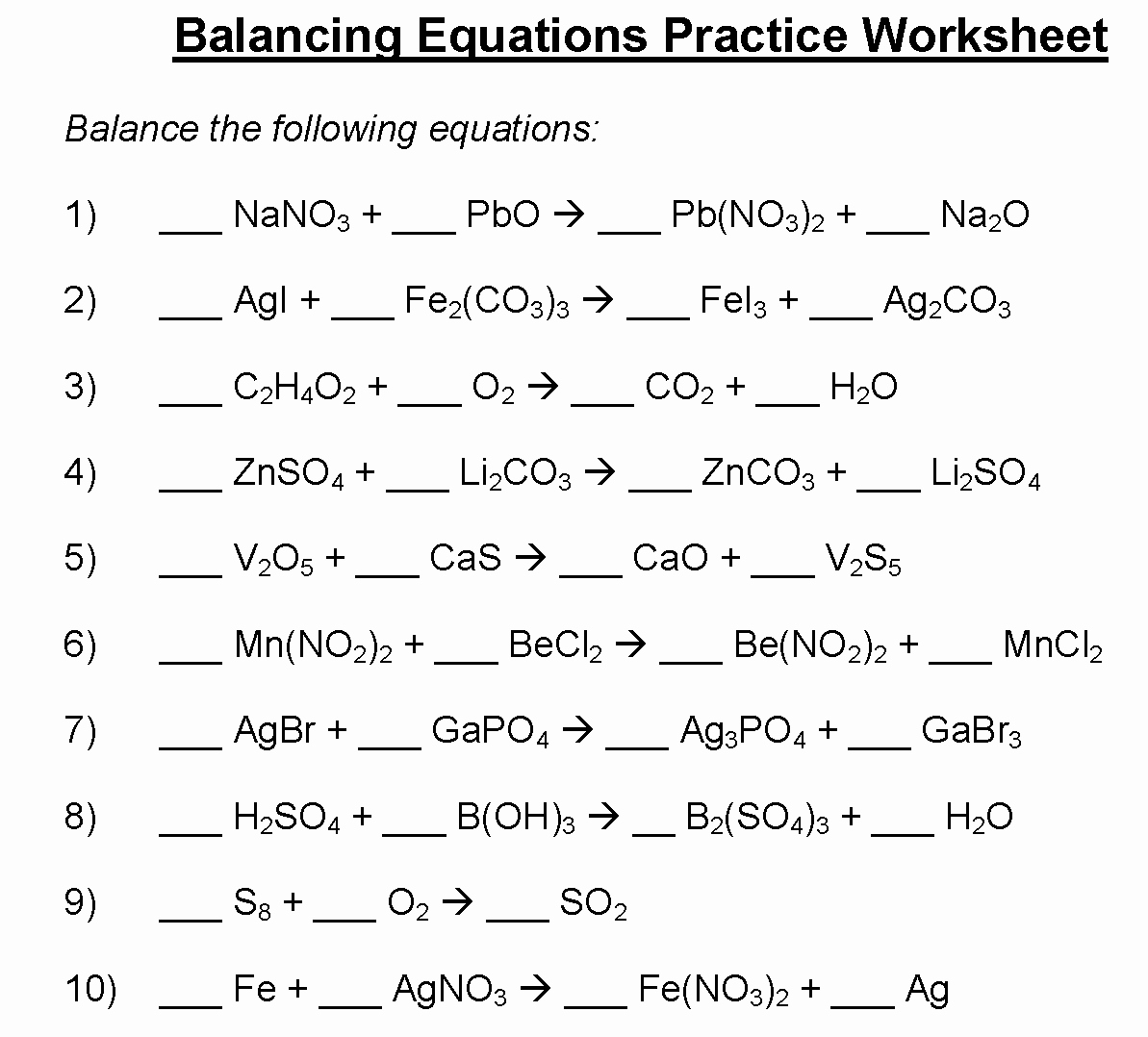
Equations balancing is a fundamental skill in chemistry that involves ensuring that the number of atoms of each element on both sides of a chemical equation is the same. This process is essential for understanding chemical reactions and predicting the products that will be formed. Balancing equations can be tricky, but with practice and the help of worksheets, it can become easier.
Equations balancing worksheets are a valuable tool for students to practice and master this important skill. These worksheets typically contain a series of chemical equations that are unbalanced, requiring students to determine the coefficients that will balance the equation. By working through these worksheets, students can develop their problem-solving abilities and gain a deeper understanding of chemical reactions.
Equations Balancing Worksheet
One common method for balancing equations is to start by balancing the atoms of elements that appear in only one reactant and one product. For example, consider the equation:
2H2 + O2 → 2H2O
In this equation, hydrogen and oxygen are the only elements that appear in one reactant and one product. By adjusting the coefficients of the reactants and products, we can balance the equation:
2H2 + O2 → 2H2O
Another strategy for balancing equations is to balance atoms that appear in multiple reactants and products. For example, consider the equation:
Fe + O2 → Fe2O3
In this equation, iron and oxygen appear in both the reactants and the products. By adjusting the coefficients, we can balance the equation:
4Fe + 3O2 → 2Fe2O3
Equations balancing worksheets provide students with the opportunity to practice these strategies and improve their skills. By working through a variety of equations, students can gain confidence in balancing chemical equations and develop a deeper understanding of chemical reactions.
In conclusion, equations balancing worksheets are an essential tool for students to practice and master the skill of balancing chemical equations. By working through these worksheets, students can improve their problem-solving abilities and gain a better understanding of chemical reactions. With practice and persistence, students can become proficient in balancing equations and succeed in their chemistry studies.